Abstract
Despite the high rates of complete remissions achievable by modern frontline therapies, relapse of acute lymphoblastic leukemia (ALL) remains common and is associated with poor long-term survival. Relapse occurs due to the outgrowth of cells from the residual leukemia niche. These are able to evade populations of leukemia-specific memory/effector CD8+ and CD4+ T-cells that are robustly detectable in most patients. This implies a need for better understanding of basic mechanisms underlying relapse and leukemic immune evasion.
The presence of phenotypically exhausted PD1+/- TIM3+ CD4+ T-cells in diagnostic bone marrow biopsy samples has been associated with inferior clinical outcomes in clinical studies of ALL patients. This suggests that CD4+ T-cell dysfunction facilitates immune escape by residual leukemic cells. To examine this, we used single-cell RNAseq/CITESEq to study CD4+ T-cells in diagnostic bone marrow biopsy samples and a murine model of BCR-ABL-rearranged ALL. In both settings, we discovered that FOXP3-PD1+ TIM3+ CD4+ T-cells are primarily composed of a unique population capable of helper and cytotoxic functions ("T-helper cytotoxic" or THCTX cells). THCTX cells expressed the effector cytokines TNF and IFNγ, but also the suppressive cytokine IL-10. In advanced leukemia, THCTX cells maintained IFNγ expression but lost the capacity for TNF synthesis, suggestive of a unique state of exhaustion. Administration of an anti-PDL1 antibody in combination with the BCR-ABL inhibitor nilotinib markedly improved long-term survival from 10% to 70% in preclinical models. Treatment benefit from dual therapy was dependent on the presence of CD4+ T-cells and was associated with the clonal expansion of leukemia-specific THCTX cells. Expanded THCTX clones had relatively preserved expression of the helper molecule CD40L as well as of ligands for the CCR5 chemokine receptor, which plays a key role in recruitment of memory/effector T-cells. Congruent with these observations, anti-PDL1 checkpoint blockade was also associated with higher frequencies of Granzyme+ CD8+ T-cells.
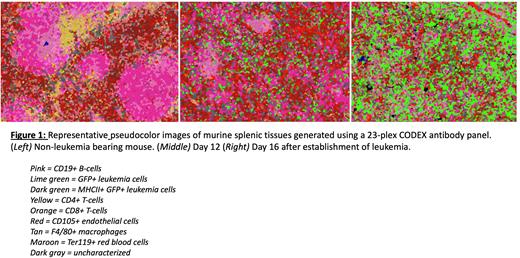
T-cell exhaustion occurs due to chronic TCR stimulation occurring in the presence of concerted microenvironmental cues from surrounding cell types. To understand how the topography of the leukemia niche contributes to the observed exhaustion of CD4+ T-cells, we performed spatial proteomic profiling of mouse splenic tissue during leukemia development. Leukemic cells express GFP and were recognized with an anti-GFP antibody. Early leukemic infiltration led to pronounced disruption of the immune structures of the spleen and the adoption of novel cell neighborhoods (Figure 1). Infiltration was marked by loss of boundaries between the red and white pulp, and dissolution of the marginal and mantle zones. CD4+ and CD8+ T-cells were diminished in frequency, infrequently observed in proximity to leukemic cells, and expressed exhaustion markers, suggestive of an immune desert phenotype.
Overall, these findings support a model in which ALL progression occurs due to the accrued dysfunction of CD4+ THCTX cells marked by a loss of helper and chemotactic functions. This is coincident with profound disruption and reorganization of the lymphoid environment into T-cell poor zones. These findings provide mechanistic insights into the high relapse risk observed among patients with CD4+ T-cell phenotypic exhaustion.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal